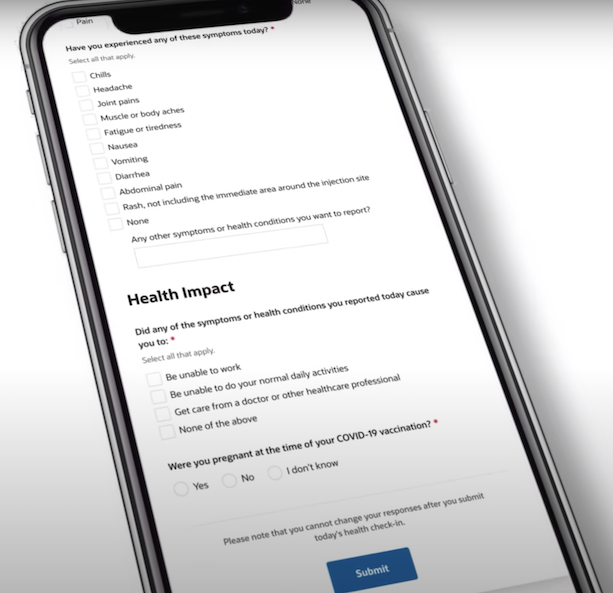
“It doesn’t have the extent of element and granularity wanted to precisely assess whether or not these occasions are causally associated. And , there’s no comparability group there, everyone in v-safe is vaccinated. And so it’s unattainable to find out whether or not a vaccine brought on a selected antagonistic occasion simply based mostly on v-safe information,” Belongia instructed us.
The system is a part of a larger early alert reporting system that enables consultants to observe the security of the vaccines. Belongia, and different consultants, say v-safe has limitations. However he says the instrument has been useful to watch widespread unintended effects of the vaccines comparable to ache on the injection web site, headache, muscle aches or fever.
“And what we’re seeing is essentially according to what was seen within the scientific trials: that these signs are quite common, they’re delicate to reasonable, they resolved over two to a few days, and so there’s actually nothing stunning there,” he mentioned.
The information collected via v-safe isn’t accessible to the general public in the best way the information from different methods just like the Vaccine Adverse Event Reporting System is. As an alternative, the knowledge is communicated to the general public via a number of research that analyze v-safe information within the CDC’s Morbidity and Mortality Weekly Report and other journals, and in displays to the Advisory Committee on Immunization Practices. Based on the CDC, v-safe information have been introduced in 11 conferences because the rollout of the vaccines — in December 2020; January, March, June, September and October 2021; and in January, April, May, June and September 2022.
Some findings of these studies are that the second dose of the first sequence brought on extra contributors to report delicate and non permanent signs or being unable to do regular exercise, and that the second booster caused fewer of the anticipated unintended effects, comparable to ache on the web site of injection, than the primary one. Based on the CDC, when the COVID-19 vaccines began to be administered, v-safe was critical in identifying early reports of a extreme allergic response that happens hardly ever after vaccinations.
One examine, printed within the Lancet Infectious Ailments in March, analyzed v-safe reviews between Dec. 14, 2020, and June 14, 2021, after the administration of the Pfizer/BioNTech and the Moderna COVID-19 vaccines. Based on its findings, less than 1% of v-safe customers reported receiving medical care within the first week after getting a dose of the first sequence of both vaccine, and a really small proportion (0.2% or fewer) reported an emergency room go to or hospitalization. Once more, even these medical care visits aren’t essentially brought on by the vaccine and could possibly be coincidental.
ICAN’s Deceptive Evaluation
Knowledgeable Consent Motion Community is a Texas-based group founded by Del Bigtree, an anti-vaccination activist. The group has filed a number of vaccine-related lawsuits towards the CDC, the Meals and Drug Administration and the Nationwide Institutes of Well being. ICAN sued the CDC in December 2021, and once more in May 2022, following FOIA requests to acquire all information submitted to v-safe since Jan. 1, 2020. The v-safe program solely began after the COVID-19 vaccines had been licensed in December 2020.
In August, the CDC agreed to publish on its web site the information collected from greater than 10 million v-safe contributors by Sept. 30. The database would cowl reviews submitted between Dec. 14, 2020, and July 31, 2022, and would omit any private identifiers. The company instructed Reuters “technical and administrative” points prevented it from publishing the information on time. As an alternative, the CDC handed the information on to ICAN, which published its personal evaluation on Oct. 3.
On its web site, ICAN does not clarify the strategies used for its evaluation. However it mentions the information are restricted to the ten million v-safe customers and to the pre-populated fields checked by them, not the knowledge customers can add in textual content containers. Based on the group’s dashboard, the information are drawn from check-ins that occurred as much as a yr after a vaccine dose. The group additionally published 5 downloadable information with uncooked v-safe information.
FactCheck.org reached out to Aaron Siri and different representatives of Siri & Glimstad, who filed the FOIA litigation for ICAN, however we didn’t hear again from them. We additionally tried to entry the uncooked information introduced, however we couldn’t open the largest file containing info relating to the well being check-ins.
We had been capable of entry one of many information containing signs and well being impacts, which included 116,294 reviews. Out of those reviews, 1,046, or 0.9%, had been reviews of receiving medical care of any form, and this included a number of reviews from the identical particular person over time. Out of these, solely seven had been for hospitalizations, two of which had been for a similar particular person on two consecutive days.
Belongia, the vaccine security professional, instructed us the numbers supplied by ICAN, together with the more than 780,000 users who reported requiring medical care of any form, are “uninterpetable.”
“We don’t know what the background fee within the inhabitants is from v-safe. What would that quantity have been in a bunch of people that hadn’t gotten the vaccine?” he mentioned.
Since well being issues, together with hospitalizations, happen within the inhabitants on daily basis, it’s not unusual for them to occur after vaccination for causes unrelated to the vaccine. That’s why it’s essential to see if a selected occasion is going on extra continuously after vaccination, which might point out a difficulty.
The evaluation doesn’t point out how lengthy after vaccination a consumer required medical consideration, however included responses as much as a yr after vaccination, which might clarify the distinction between ICAN’s numbers and the CDC’s printed information. Siri told Reuters that ICAN thought it was essential to look past one week, since some potential vaccine-related unintended effects might seem weeks after vaccination. However most unintended effects happen quickly after vaccination, so together with longer intervals would come with extra occasions which might be unrelated to the vaccine.
A consumer could have gone to a physician for a totally completely different cause six months after a vaccine, and, if following v-safe directions correctly, would report it to the system. That consumer would get a follow-up name from v-safe, for the company to get extra info. However as we mentioned, v-safe doesn’t have the flexibility to know if any specific occasion, together with a hospitalization, was brought on by the vaccine or not.
“And so with a view to reply questions like that, when it comes to relative danger, you want one thing just like the Vaccine Security DataLink or VSD, which makes use of medical information from hundreds of thousands of individuals across the nation, and immunization information to implement scientifically legitimate research to find out if there’s an elevated danger after vaccination for specific antagonistic occasions,” Belongia mentioned, referring to a network of nine integrated health care organizations, together with his, that conducts lively vaccine security surveillance.
“What we’re truly seeing within the information is the precise reverse of what’s being urged by teams like ICAN — these vaccines are very protected. With, , a couple of very uncommon identified exceptions,” Belongia mentioned. “However total, it’s very clear that the advantages of the vaccines enormously outweigh the dangers.”
Editor’s observe: SciCheck’s COVID-19/Vaccination Project is made doable by a grant from the Robert Wooden Johnson Basis. The inspiration has no control over FactCheck.org’s editorial choices, and the views expressed in our articles don’t essentially mirror the views of the inspiration. The purpose of the undertaking is to extend publicity to correct details about COVID-19 and vaccines, whereas reducing the impression of misinformation.
Sources
COVID Data Tracker. CDC. Accessed 27 Oct 2022.
Selected Adverse Events Reported after COVID-19 Vaccination. CDC. Up to date 24 Oct 2022. Accessed 27 Oct 2022.
V-safe After Vaccination Health Checker. CDC. Up to date 18 Jul 2022. Accessed 27 Oct 2022.
Sparber, Sami. “Texas-based anti-vaccine group received federal bailout funds in May as pandemic raged.” The Texas Tribune. 18 Jan 2021.
“BREAKING NEWS: ICAN OBTAINS CDC V-SAFE DATA.” ICAN. 3 Oct 2022.
Rosenblum, Hannah G., et al. “Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe.” Lancet Infectious Ailments. 22 Jun 2022.
Belongia, Edward. Director of the Middle for Medical Epidemiology and Inhabitants Well being on the Marshfield Clinic Analysis Institute. Telephone interview with FactCheck.org. 27 Oct 2022
“Share your COVID-19 vaccination experience with v-safe.” CDC. You Tube. 21 Mar 2021.
COVID-19 Vaccine Reporting Systems. CDC. Accessed 27 Oct 2022.
Michelle Gomez, Amanda. “V-Safe: How Everyday People Help the CDC Track Covid Vaccine Safety With Their Phones.” KHN. 7 Sep 2021.
Hause, Anne M., et al. “Safety Monitoring of Pfizer-BioNTech COVID-19 Vaccine Booster Doses Among Children Aged 5–11 Years — United States, May 17–July 31, 2022.” MMWR. 19 Aug 2022.
Hause, Anne M., et al. “Safety Monitoring of COVID-19 mRNA Vaccine Second Booster Doses Among Adults Aged ≥50 Years — United States, March 29, 2022–July 10, 2022.” MMWR. 29 Jul 2022.
Hause, Anne M., et al. “Safety Monitoring of COVID-19 Vaccine Booster Doses Among Adults — United States, September 22, 2021–February 6, 2022.” MMWR. 18 Feb 2022.
Hause, Anne M., et al. “Reactogenicity of Simultaneous COVID-19 mRNA Booster and Influenza Vaccination in the US.” JAMA Community Open. 18 Feb 2022. 1 Jul 2022.
ACIP Meeting Information. CDC. Accessed 27 Oct 2022.
CDC COVID-19 Response Workforce; Meals and Drug Administration. “Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14–23, 2020.” MMWR. 15 Jan 2021.
Greene, Jenna. “New data is out on COVID vaccine injury claims. What’s to make of it?” Reuters. 12 Oct 2022.
Vaccine Safety Datalink (VSD). CDC. Accessed 27 Oct 2022.
Sharan, Martha. CDC Media Relations. E-mail to FactCheck.org. 25 Oct 2022.






